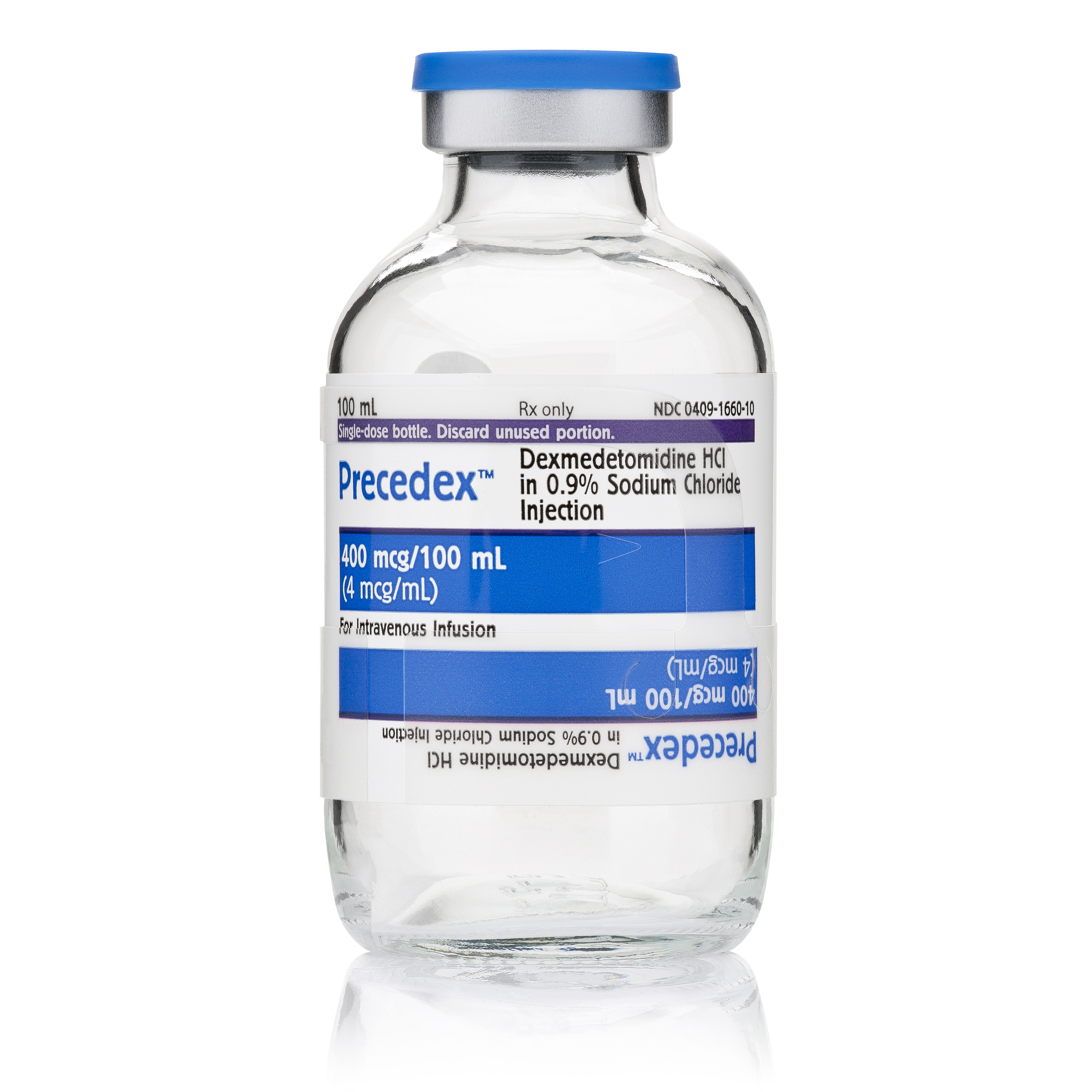
Precedex™ (dexmedetomidine hydrochloride in 0.9% sodium chloride injection)
4 mcg/mL
400 mcg/100 mL
Glass Bottle
Unit of Sale NDC: 00409-1660-10
| Wholesalers | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardinal | 4844882 | ||||||||||||||||||
| Cencora | 10116803 | ||||||||||||||||||
| McKesson | 1907542 | ||||||||||||||||||
| Morris & Dickson | 127977 | ||||||||||||||||||
| Ordering information | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit of Sale size | 1 Case (10 Bottles) | ||||||||||||||||||
| Case size | 1 x 10 | ||||||||||||||||||
| Cases per tier | 32 | ||||||||||||||||||
| Cases per pallet | 256 | ||||||||||||||||||
| Allergy information | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-DEHP | |||||||||||||||||||
| Latex information | |||||||||||||||||||
| Preservative free | |||||||||||||||||||
| Gluten free | |||||||||||||||||||
| Availability specifics | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Additional information | View | ||||||||||||||||||
If Latex Information is present above, then natural rubber latex has not been used in the manufacturing of this device or drug container closure system.
If Preservative Free is present above, then this product does not contain ingredients identified as a preservative, as defined by the USP.
Cap color: Blue