Filters
UoS = Unit of Sale
Depleted
To set product alerts you must
or register
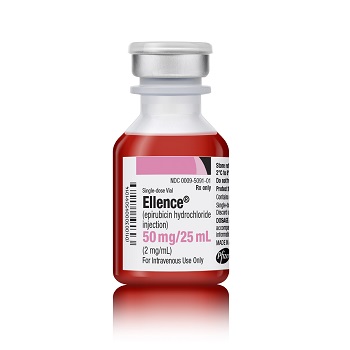
Ellence® (epirubicin hydrochloride injection)
50 mg
CYTOSAFE® Single-use Vial
UoS NDC: 00009-5091-01
Next Delivery:
12/2027
12/2027
Est. Recovery:
4Q 2027
4Q 2027
Available
To set product alerts you must
or register
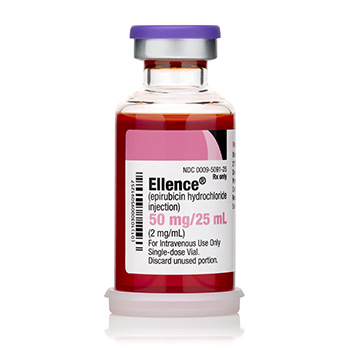
Ellence® (epirubicin hydrochloride injection)
50 mg/ 25 mL
Onco-Tain™ Single Dose Glass Fliptop Vial
UoS NDC: 00009-5091-25
Available
To set product alerts you must
or register
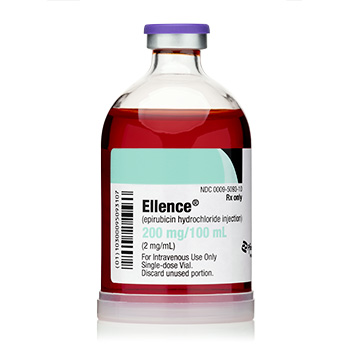
Ellence® (epirubicin hydrochloride injection)
200 mg/ 100 mL
Onco-Tain™ Single Dose Glass Fliptop Vial
UoS NDC: 00009-5093-10
