Filters
UoS = Unit of Sale
Available
To set product alerts you must
or register
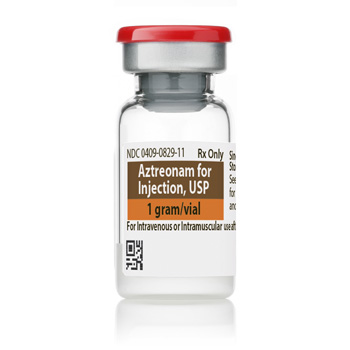
Aztreonam for Injection, USP
1 g
Vial
UoS NDC: 00409-0829-01
Available
To set product alerts you must
or register
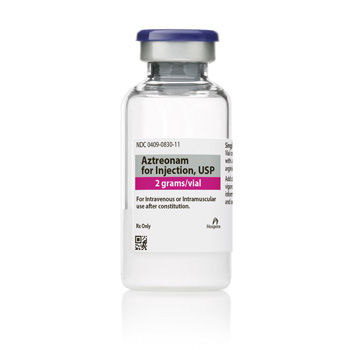
Aztreonam for Injection, USP
2 G
Vial
UoS NDC: 00409-0830-01
